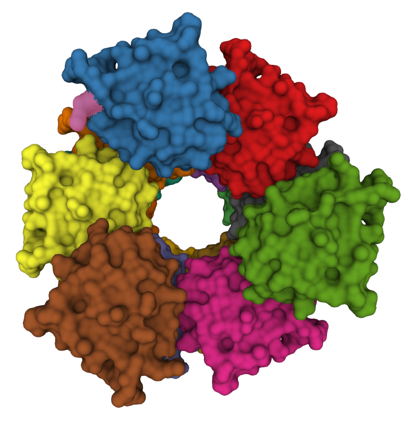
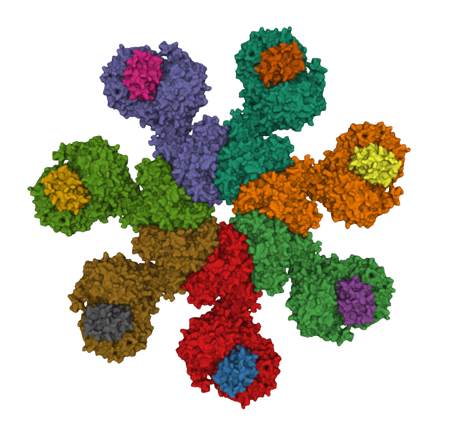
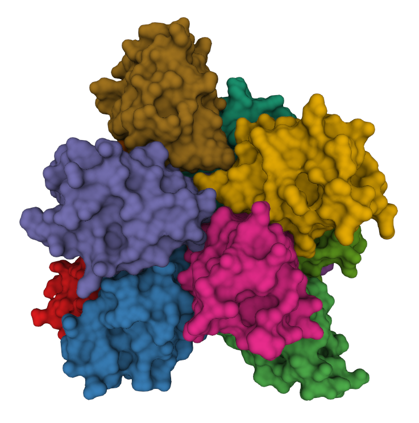
Apoptosis plays key roles in development, immunity, and in removing potentially cancerous cells. It is also dysregulated in a range neuro-degenerative diseases. Apaf-1, a key protein in the mitochondrial apoptosis pathway, is normally kept in an inactive conformation by intramolecular interactions between its nucleotide binding domain (NBD) and WD domains. Cytochrome c binding to the WD domains disrupts NBD-WD interactions, allowing Apaf-1 to oligomerize into the apoptosome. The apoptosome then recruits and activates caspase-9 via CARD-CARD interactions. Active caspase-9 then cleaves and activates caspase-3, which in its turn brings about the morphological and biochemical changes of apoptosis. Other apoptotic pathways also involve the formation of large protein complexes; for example a variety of intracellular stresses, including DNA damage and chemotherapy, activate caspase-2 by triggering formation of the PIDDosomea complex. The death receptor pathways involve formation of the Death Inducing Signalling Complex or DISC in response to FasR activation. TNF-a activation of TNF-Receptor 1 is typically a pro-survival signal, however, in some contexts cell-death inducing protein complexes (called “IIa”, “IIb” and the “necroptosome”) form, leading either to apoptosis or necroptosis.
Necroptosis is a form of cell death activated during inflammation and in response to viral infection. RIPK1 and RIPK3 complexes are involved in necroptosis. Various stress induced signals or cytokines can induce interactions between the RHIM domains of the RIPKs and result in activation of RIPK3, which then goes on to phosphorylate MLKL. MLKL then oligomerizes to form a complex that permeabilizes the plasma membrane. RIPK3 has also been implicated in several other types of regulated necrosis.
Pyroptosis is a form of cell death activated by pathogens. NLRP3 is a protein involved in recognizing Pathogen Associated Molecular Patterns and mitochondrial uncoupling. NLRP3 oligomerizes to form the NLRP3 inflammasome which binds proteins ASC and caspase-1. This in turn leads to caspase-1 activation and the regulation of inflammation through cleavage of IL-1β. Caspase-1 causes pyroptotic cell death by cleaving Gasdermin D, which can then makes a pore in the cell’s plasma membrane, causing a necrotic cell death.
Visualizing cell-death inducing protein complexes
We have developed split-luciferase complementation assays to detect the apoptosome and the inflammasome. Our goal now is to develop new luminescent and fluorescent tools that will allow us to visualize death-inducing complexes in cells.